Research Projects
1.) The disulfide relay system in the intermembrane space of mitochondria
We identified a novel mechanism of protein import into mitochondria. We identified in the intermembrane space of mitochondria a disulfide relay system consisting of the redox-regulated receptor Mia40 and the thiol oxidase Erv1. This system introduces disulfide bonds in cysteine-rich proteins and thereby drives their import into the intermembrane. We want to understand how this systems transfers disulfide bonds into the substrate proteins. Moreover, we aim to identify additional substrates of the Mia40-Erv1 system and to analyse the effects of the disulfide relay system on the redox homeostasis of the intermembrane space.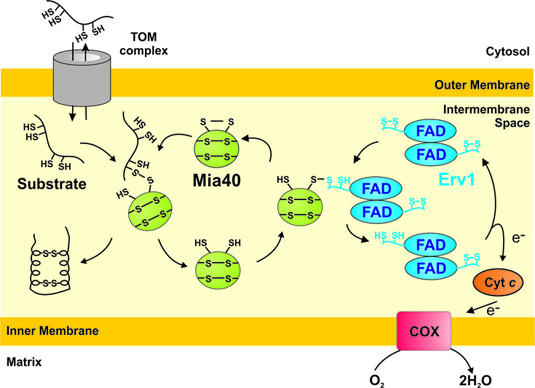
2.) Structure und function of the TIM23 preprotein translocase and the mitochondrial import motor
The TIM23 preprotein translocase is the main translocase of the inner membrane. It mediates the transport of presequence containing proteins in a membrane potential and ATP dependent manner into the matrix and the inner membrane of mitochondria. Besides the receptor and pore forming subunits, the TIM23 translocase contains subunits of the mitochondrial import motor. The import motor is associated with the integral components of the TIM23 translocase at the matrix side of the inner membrane and promotes complete ATP dependent transport of preproteins across the translocation pore. Our goal is to understand the mechanism and function of this translocase and its import motor. We employ biochemical, biophysical and genetic methods to elucidate how the components of the translocase interact and function to mediate translocation of preproteins
3.) Structural and functional characterisation of the mitochondrial chaperone Hep1
We have identified a mitochondrial Hsp70 (mtHsp70) interacting protein, the Hsp70 escort protein (Hep1), which prevents the aggregation of mitochondrial Hsp70 chaperones. Loss of Hep1 has severe implication for the cell and leads to a variety of mitochondrial defects. The molecular mechanisms of the function of Hep1 are, however, not understood. It is our aim to understand why mitochondrial Hsp70 chaperones have the propensity to aggregate and how this novel kind of chaperone protects them.

