Research Projects
Previous work
The conserved and essential histone chaperone FACT (Facilitating Chromatin Transactions) is frequently overexpressed in cancer. In recent years, we have investigated FACT’s functions in both active and silent regions of the genome. Our studies have shown that H2B monoubiquitination modulates FACT activity at transcribed genes. We also discovered that FACT contributes to the compaction of subtelomeric chromatin structures, which are important for meiotic gene silencing (Murawska et al., Mol Cell, 2020). More recently, we identified FACT as a unique histone chaperone that promotes heterochromatin spreading along chromosomal arms—a process essential for maintaining gene expression patterns and the faithful inheritance of epigenetic states (Murawska et al., Cell Rep, 2021).
Current projects
1. Chromatin dynamics regulation by the FACT chaperone
We continue our studies on the FACT chaperone with a special focus on less understood, transcription-independent processes.
We aim to answer the following questions:
• What is the role of FACT at centromeres and in chromosome segregation?
• How is FACT’s stability and turnover regulated across the genome?
• How does FACT coordinate with other histone chaperones during histone hand-off?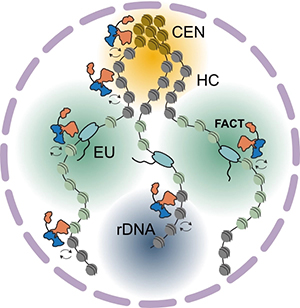
2. Nuclear functions of bromodomain AAA+ ATPases
AAA+ ATPases (ATPases Associated with diverse cellular Activities) are molecular machines involved in protein complex remodeling and cellular quality control. Bromodomain-containing AAA+ ATPases are a poorly understood subgroup and are considered putative atypical histone chaperones with emerging links to cancer. Despite growing interest in targeting their bromodomains, little is known about their chromatin-related functions.
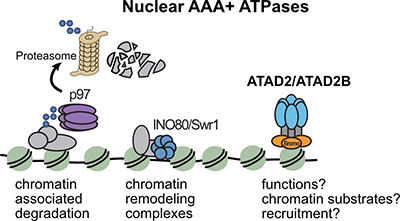
Our current work aims to determine:
• What are the chromatin substrates of these ATPases?
• What are their enzymatic activities?
• How are they recruited to chromatin?
• Which molecular pathways do they regulate?

